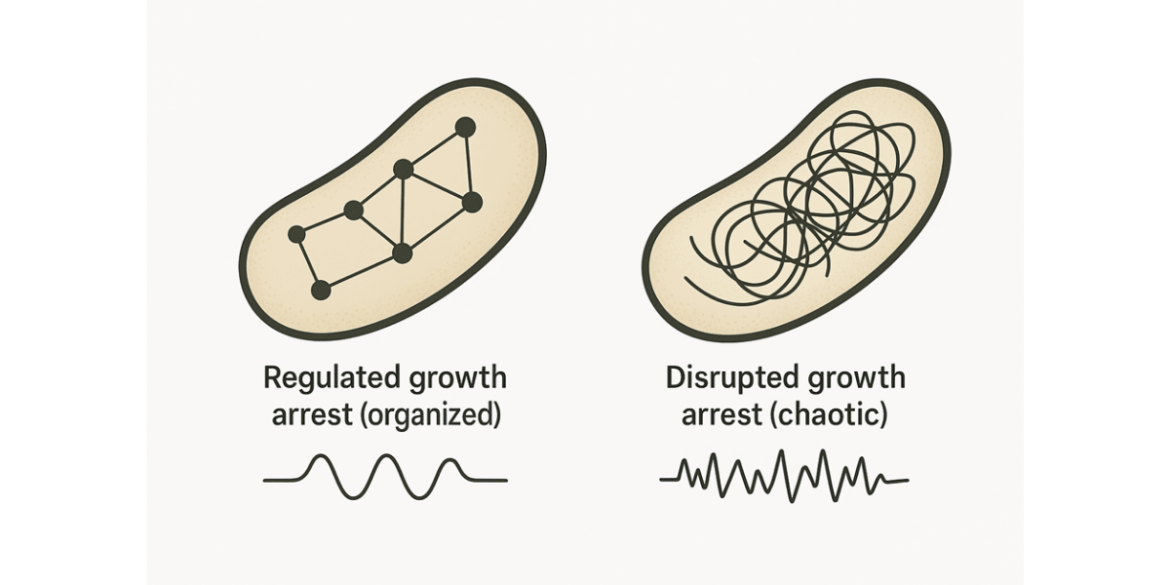
A new study from the Hebrew University reveals that bacteria can survive antibiotic treatment through two fundamentally different “shutdown modes” – not just the classic idea of dormancy. In one, cells enter a regulated, protective growth arrest: a controlled, dormant state that shields them from antibiotics that target actively growing bacteria. In the other, they survive in a disrupted, dysregulated growth arrest: a malfunctioning state marked by clear weaknesses, especially unstable cell membranes.
This distinction matters because antibiotic persistence is a major cause of treatment failure and relapsing infections even when bacteria are not genetically resistant. By showing that persistence can arise from two distinct biological states, the study helps resolve years of conflicting results and points towards more precise treatment strategies that target each persister type differently.
Antibiotics are meant to wipe out harmful bacteria. Yet in many stubborn infections, a small fraction of bacterial cells manage to endure treatment, only to re‑emerge later and cause relapse – a phenomenon known as antibiotic persistence. For years, this has largely been blamed on bacteria “going to sleep” to evade drugs that attack growing cells. But new research led by PhD student Adi Rotem under the supervision of Prof. Nathalie Balaban at the Hebrew University shows that this explanation is only half the picture.
The team demonstrated that high survival under antibiotics can arise from two qualitatively different growth‑arrest states – and they are not just shades of the same “sleeping” behaviour. One is a controlled, regulated shutdown, the classic dormancy model. The other is a disrupted, dysregulated arrest, in which bacteria survive not through protective calm but by entering a malfunctioning state with distinct vulnerabilities. “We found that bacteria can survive antibiotics by following two very different paths,” said Prof. Balaban. “Recognising the difference helps resolve years of conflicting results and points to more effective treatment strategies.”
Two Survival Modes – And Why They Matter
The researchers identified two archetypes of growth arrest that can both lead to persistence, but for very different reasons:
Regulated growth arrest: a protected dormant state
In this mode, bacteria actively slow down and enter a stable, defended condition. These cells are difficult to kill because many antibiotics rely on active bacterial growth to work.Disrupted growth arrest: survival through breakdown
In the second mode, bacteria enter a dysregulated, disrupted state. This is not a planned shutdown but a loss of normal cellular control. These bacteria show broad impairment in membrane homeostasis – a core function needed to maintain cell integrity. That weakness could become a key treatment target.
A New Framework for Smarter Antibiotic Strategies
Antibiotic persistence contributes to recurring infections in many settings, from chronic urinary tract infections to infections associated with medical implants. Despite intensive research, scientists have struggled to agree on a single mechanism explaining why persister cells survive; different experiments have produced conflicting pictures of what persisters look like and how they behave. This study offers a unifying explanation: researchers may have been observing different types of growth‑arrested bacteria without realising they were distinct.
By separating persistence into two different physiological states, the findings suggest a future in which treatments can be tailored – targeting dormant persisters one way and disrupted persisters another. In principle, this could make it far harder for infections to “bounce back” after antibiotic courses.
How the Researchers Saw What Others Missed
To tease apart these hidden states, the team combined mathematical modelling with several high‑resolution experimental tools, including:
Transcriptomics, to track how bacterial gene expression shifts under antibiotic stress
Microcalorimetry, to follow metabolic changes via tiny heat signals
Microfluidics, to observe single bacterial cells under tightly controlled conditions
Together, these approaches revealed clear biological signatures that distinguish regulated growth arrest from disrupted growth arrest, along with the specific vulnerabilities associated with the disrupted state.
Funding
European Research Council Advanced Grant, grant no. 101054653
The Minerva Center for Stochastic Decision Making in Microorganisms
Israel Science Foundation, grant no. 597/20
The Milner Foundation, founded by Yuri Milner and his wife Julia (supporting AR)
The research paper, “Differentiation between regulated and disrupted growth‑arrests allows tailoring of effective treatments for antibiotic persistence”, is published in Science Advances and is available at: 10.1126/sciadv.adt6577.
Researchers
Adi Rotem, Yoav Kaplan, Orit Gefen, Irine Ronin, Alon Gutfreund, Hagai Rappeport, Raya Faigenbaum‑Romm, Nitsan Naor, Elisheva Stav, Oded Agam, Nathalie Q. Balaban
Institution
Racah Institute of Physics, The Hebrew University of Jerusalem
