Experimental approach reduced seizures and helped protect brain function in preclinical studies
A new study suggests that an experimental peptide developed at the Hebrew University may reduce recurring seizures and support brain function by targeting underlying oxidative stress and inflammation processes linked to epilepsy. Unlike current treatments that focus mainly on suppressing seizures, this approach could influence how the disease develops over time, with the greatest benefits appearing when treatment begins early. The findings point to a promising direction for future therapies aimed at improving long-term outcomes for people living with epilepsy.
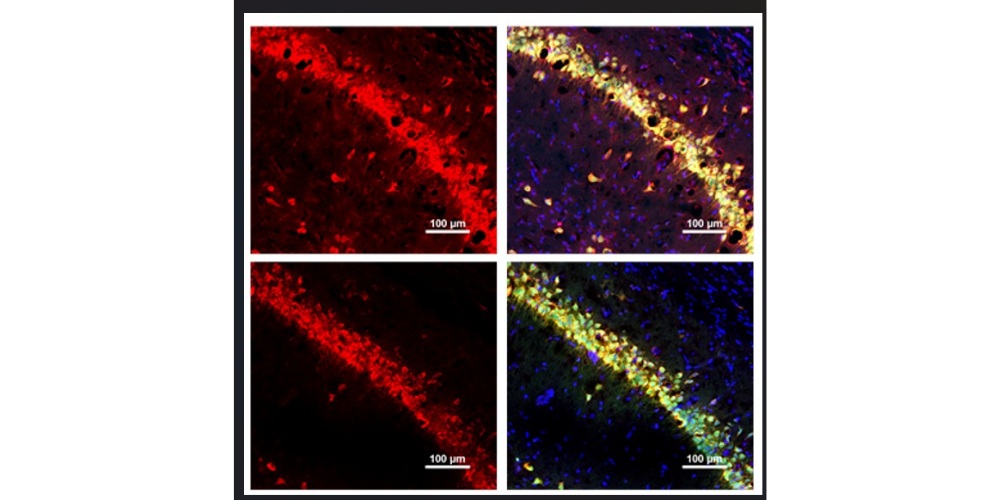
Title image: TXM-CB3 reduces oxidative damage.
Red fluorescence indicates oxidative damage. The upper panel shows untreated tissue, while the lower panel shows tissue following TXM-CB3 treatment, demonstrating a marked reduction in fluorescence intensity and extent of oxidative damage after TXM-CB3 administration. Credit: Prince Kumar Singh, Shweta Maurya
Epilepsy is one of the most common neurological disorders in the world. According to the World Health Organisation, around 50 million people live with epilepsy, a condition marked by recurring seizures that can also affect mood, memory, and day-to-day thinking. While many medications can reduce seizures, up to 40 per cent of patients don’t respond well enough, and today’s drugs generally don’t stop the condition from worsening over time.
A new study from the Hebrew University of Jerusalem suggests a different approach: treating epilepsy by calming harmful chemical and immune “stress signals” in the brain that may help seizures keep returning and may contribute to longer-term damage.
The research, led by PhD students Prince Kumar Singh and Shweta Maurya, under the guidance of Prof Tawfeeq Shekh-Ahmad of the School of Pharmacy, Faculty of Medicine, in collaboration with Prof. Daphne Atlas of the Alexander Silberman Institute for Life Sciences, was published in Redox Biology. The study focused on the experimental compound TXM-CB3, a member of a family of low-molecular-weight thioredoxin-mimetic peptides designed by Daphne Atlas, who showed its protective effects against cognitive impairment induced by mild traumatic brain injury and in attenuation of allergic airway disease. This tiny tripeptide is designed to imitate the activity of a natural protective protein in the body known as thioredoxin.
Thioredoxin is part of the body’s built-in defense system: it helps cells cope with chemical strain and helps regulate inflammation. Both processes are increasingly believed to play a key role in epilepsy, not only in triggering seizures, but in shaping how the condition develops and becomes harder to treat.
“Most epilepsy treatments focus on reducing seizures, but our goal was to see whether we could affect the underlying processes that may drive the disease forward,” said Prof Shekh-Ahmad.
What the researchers found
In early experiments using nerve-cell models that produce seizure-like activity, TXM-CB3 reduced signs of damaging chemical strain and shifted the balance of immune signals away from an aggressive, inflammatory pattern and toward a more protective one.
The researchers then tested the treatment in preclinical models designed to mirror severe, recurring seizures in drug-resistant epilepsy. They examined two treatment windows that matter clinically:
Early treatment: soon after a major seizure event:
When TXM-CB3 was given early, seizures began later and happened less often. The overall seizure “load” was lower, and brain regions important for memory were better preserved. The treatment was also linked with improvements in behavior, including lower anxiety-like behavior and better performance on a short-term memory task.
Later treatment: after recurring seizures were already established:
Even when treatment started later, TXM-CB3 continued to reduce seizure activity over time. However, thinking and memory problems that had already developed did not substantially improve. This result highlights the potential value of early intervention.
“The fact that we saw both reduced seizure activity and signs of brain protection in these experimental models strengthens the case for developing treatments that build on the body’s own protective pathways,” said Prof Atlas.
Why this matters
Current epilepsy drugs primarily aim to suppress seizures in the moment. This study points to a strategy that may go further, reducing the biological conditions in the brain that can help seizures return again and again, and that may contribute to longer-term effects like anxiety and cognitive difficulties.
The researchers emphasise that the findings come from experimental models, and further studies are needed to assess safety, dosing, and effectiveness in humans. Still, the results offer a promising direction toward therapies that could one day improve not only seizure control, but long-term quality of life for people living with epilepsy.
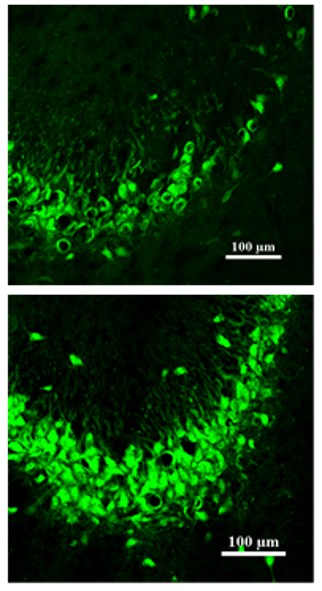
Image below: TXM-CB3 attenuates seizure-induced neuronal cell death.
Description: Green dots represent neuronal cells. Epileptic seizures induce pronounced neuronal cell loss. The upper panel shows a brain region from a rat experiencing seizures without treatment, whereas the lower panel shows the corresponding region following TXM-CB3 treatment, demonstrating preservation of neuronal cells after TXM-CB3 administration. Credit: Prince Kumar Singh, Shweta Maurya
The research paper titled “Thioredoxin-mimetic peptide attenuates epilepsy progression and neurocognitive deficits” is now available in Redox Biology and can be accessed at https://www.sciencedirect.com/science/article/pii/S2213231726000194
Researchers:
Prince Kumar Singh, Shweta Maurya1, Aseel Saadi, Sereen Sandouka, Taige Zhang, Orya Kadosh1, Yara Sheeni, Valeria Martin, Daphne Atlas, Tawfeeq Shekh-Ahmad
Institutions:
- The Institute for Drug Research, The School of Pharmacy, Faculty of Medicine, The Hebrew University of Jerusalem
- The Alexander Silberman Institute of Life Science, The Hebrew University of Jerusalem
